Quaternary Structure of Human Hemoglobin Is Best Described as
Double-click on one of the gray heme group atoms in the model above and zoom in to examine it. Hemoglobins Protein Structure Basics 1 Hemoglobin is composed of multiple protein chains.
What Is The Structure Of Hemoglobin And How Is Oxygen Bound To It Quora
Perhaps the best studied is the tense-state T 2 to relaxed-state R transition in human hemoglobin Hb an αβ 2 tetrameric oxygen transport protein in red blood cells Fig.

. The liganded hemoglobin Hb high-salt crystallization condition described by Max Perutz has generated three different crystals of human adult carbonmonoxy hemoglobin COHbA. A monomer dimer trimer tetramer or. Oxy- and deoxy-Hb were prepared from COHb by photolysis on ice under a stream of oxygen or argon respectively essentially as described in the literature.
Thus hemoglobin binds four O 2 molecules. The structure is found to be a dynamic. Large-scale conformational changes are thought to underlie the biological functions of many proteins.
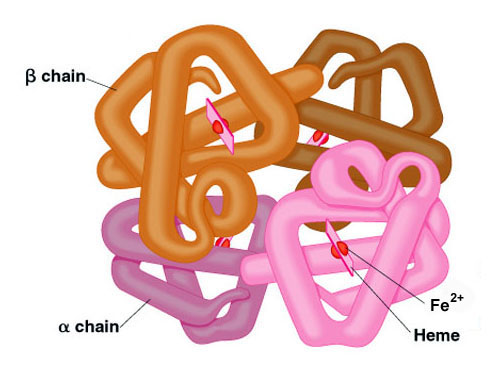
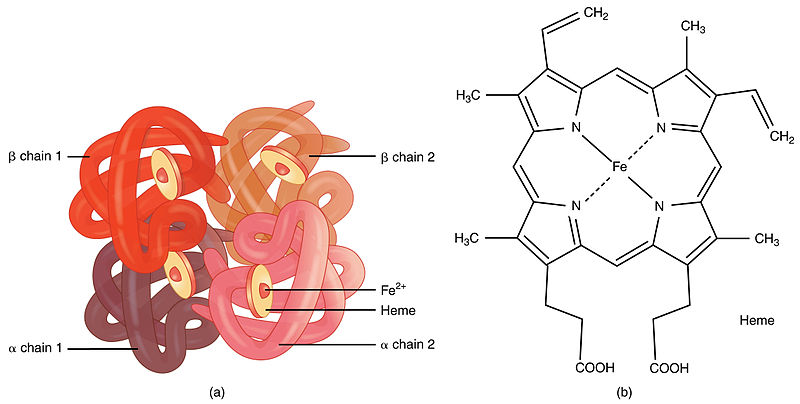
Here we demonstrate that. Solution structure cannot be excluded. The quaternary structure of hemoglobin includes the assembly of two α and two β subunits each containing a heme group that is essential for oxygen binding.
Hemoglobin has a quaternary structure. A tertiary protein will commonly contain a single polypeptide chain with one or more secondary structures. Click on the.
The peak-to-trough magnitude of the Soret band photolysis difference spectrum measured as a function of the delay between excitation pulses showed that the room temperature kinetics of geminate recombination in adult human hemoglobin are best described by two exponential processes with lifetimes of 36 and 162 ns. When two or more polypeptide chains come together to give a protein its ultimate shape that structure is described as the quaternary. An example of a protein with quaternary structure is hemoglobinIn hemoglobin one protein binds to.
The use of hydrophobic reagents and salts on the molecular weight behavior of subunit proteins such as the four-chain human and the multichain earthw. Up to 10 cash back Hemoglobin is a tetramer that possesses a quaternary structure containing multiple folded polypeptide structures tertiary structures. Which of the following statements best describes the shape and location of the heme groups.
Examples of proteins with quaternary structure are. The function of myoglobin is oxygen storage in muscle. The quaternary structure of tetrameric human normal adult car-bonmonoxy-hemoglobin can readily be determined in solution at near-physiological conditions of pH ionic strength and tempera-ture by NMR measurement of 15N-1H residual dipolar couplings in weakly oriented samples.
The structure is found to be a dynamic. The number of subunits in an oligomeric complex is described using names that end in -mer Greek for part subunit. Human hemoglobin Hb is a benchmark protein of structural biology that shaped our view of allosterism over 60 years ago with the introduction of the MWC model based on Perutz structures of the.
Proteins with Quaternary Structure. The basic properties of collagen are rigidity and resistance to stretching. There are 141 and 146 amino acids in the α and β chains of hemoglobin respectively.
Viewing the structure of human hemoglobin 1A3N with each bond in the protein drawn as a line bonds only view. It consists of two pairs of different proteins designated the α and β chains. The global chain contains an extensive alpha helix structure.
The first crystal is isomorphous with the classical liganded or R Hb structure. Monomer dimer trimer tetramer or pentamer. The quaternary structure of this protein complex would be described as a homo-trimer because it is composed of three identical smaller protein subunits or monomers.
The structure of normal adult hemoglobin can be described as____. The quaternary structure of tetrameric human normal adult car-. This structure is shown in the graphic on the left.
Best describes the structure of hemoglobin. 39 Hb concentration was assessed by visible. Hemoglobin is made up of four monomeric subunits each of which is known as a polypeptide and about the size of many normal individual proteins.
How many protein chains does hemoglobin have. The quaternary structure of collagen consists of three left-handed helices twisted into a right-handed coil. Therefore hemoglobin is said to have a quaternary structure see Reference page.
- two alpha and two beta chains. Here we demonstrate that the quaternary structure of tetrameric human normal adult carbonmonoxy-hemoglobin can readily be determined in solution at near-physiological conditions of pH ionic strength and temperature by NMR measurement of 15 N-1 H residual dipolar couplings in weakly oriented samples. As in myoglobin each subunit is linked covalently to a molecule of heme.
Each of these subunits has its. Only occurs in proteins that are made up of two or more polypeptide chains and refers to the way the multiple subunits are held together in a multi-subunit complex. The iron of the heme group is in the Fe2 oxidative state.
Hemoglobin A Hb was purified from human blood as described previously 38 and stored in the carbonmonoxy form COHb at -80 C. Which term best describes hemoglobin. In the top menu click Reset Reset structure.
1 aThe textbook description is that of a large quaternary structural. Tertiary and quaternary structure are formed by the bending and folding of. The diameter of the iron ion decreases upon binding to oxygen.
The second crystal reveals a new liganded Hb quaternary structure RR2 that assumes an.
Hemoglobin And Myoglobin The Medical Biochemistry Page

The Quaternary Structure Of Hemoglobin And Its Oxygen Carrier Heme Download Scientific Diagram
No comments for "Quaternary Structure of Human Hemoglobin Is Best Described as"
Post a Comment